I Oxygen Classes AMU.B.TECH ( 2021-21 ) Rank 07 Rank 110. Bank Medical Road, Aligarh. I Oxygen Classes. Name: Oxygen Symbol: O Atomic Number: 8 Atomic Mass: 15.9994 amu Melting Point:-218.4 °C (54.750008 K, -361.12 °F) Boiling Point:-183.0 °C (90.15 K, -297.4 °F) Number of Protons/Electrons: 8 Number of Neutrons: 8 Classification: Non-metal Crystal Structure: Cubic Density @ 293 K: 1.429 g/cm 3 Color: colorless Atomic Structure.
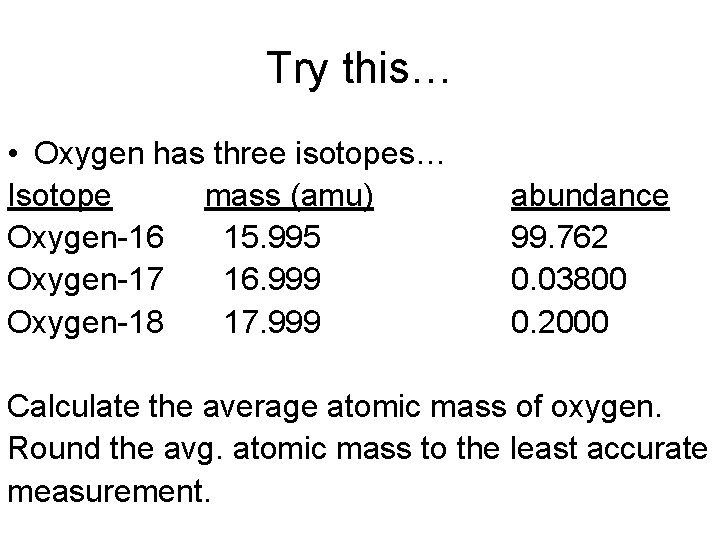
Oxygen is composed of three isotopes 16/8 O (15.995 u), 17/8 O (16.999 u) and 18/8 O (17.999 u). One of these isotopes, 17/8 O, comprises of 0.037% of oxygen. What is the percentage abundance of the other two isotopes, using the average atomic mass of 15.9994 u.

1 Answer
The abundance of
Assume you have 100 000 atoms of O. Then you have 37 atoms of
Let x = the number of atoms of
The total mass of 100 000 atoms is
x × 15.995 u + (99 963 – x) × 17.999 u + 37 × 16.999 u = 100 000 × 15.9994 u

15.995 x + 1 799 234.037 – 17.999 x + 628.963 = 1 599 940
Mass Of Oxygen Amu
2.004 x = 199 123
x = 199 123/2.004 = 99 762
So there are 99 762 atoms of
Oxygen's Amu
The number of
Hope this helps.
Related questions
