Yellowstone National Park’s geysers, hot springs, fumaroles and other hydrothermal features spew out a collection of gases from deep within the Earth—steam, carbon dioxide, methane, neon, argon and helium. There’s not enough of that last one, helium, for the park to start selling balloons or for visitors to sound like chipmunks, but there’s plenty for scientists to study.
The Helium 4 is the most advanced kite in our range. High-performance foilborders, surfers, travelers, and freeriders that want to get out in lighter wind conditions or who want a light and responsive kite will find the new Helium exceeding their expectations.
- HNT Price Live Data. The live Helium price today is $12.75 USD with a 24-hour trading volume of $27,239,850 USD. Helium is down 2.25% in the last 24 hours. The current CoinMarketCap ranking is #92, with a live market cap of $1,013,242,415 USD.
- Helium Black is designed with premium materials for all golfers looking for a lightweight shaft structure with extreme stability.
- Detailed decay information for the isotope helium-4 including decay chains and daughter products.
- Definition of helium-4 in the Definitions.net dictionary. Meaning of helium-4. What does helium-4 mean? Information and translations of helium-4 in the most comprehensive dictionary definitions resource on.
Related Content
Helium can bubble out of volcanic rocks that drive hydrothermal activity, but that’s not where most of Yellowstone’s helium is coming from, it seems. The park’s gas originates deep in rocks where it’s been stored for hundreds of millions of years, U.S. Geological Survey scientists report today in Nature.


Helium is the second-most abundant element in the universe—it’s formed by the nuclear fusion of hydrogen atoms, a process that powers stars—but it’s pretty rare here on Earth. Lucky for birthday-party goers and clowns (and modern medicine), helium can be extracted from reserves of natural gas underground.
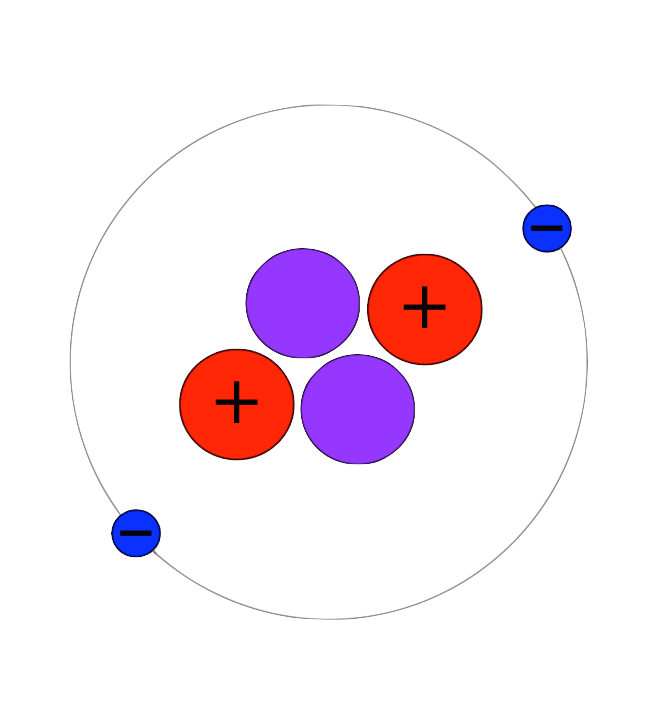
Helium on Earth can be found in two main forms: Nearly all occurs as helium-4 (named thus because it has two protons and two neutrons), which can be produced during the radioactive decay of heavy elements such as uranium. A tiny fraction (about one in a million) occurs as helium-3 (two protons and one neutron), most of which has been present on Earth since the planet’s formation and is a vestige of material that originally formed the planet.
The ratio of helium-3 to helium-4, though, slightly varies and scientists can actually use that ratio to determine the approximate source of any helium they find. Uranium and other heavy elements are more incompatible with minerals found in the Earth's mantle, so these elements filter up to the crust, where their decay produces helium-4. Mantle materials, which contain less of these heavy radioactive elements, don't produce as much helium-4, so the helium present there is mostly in the form of helium-3.
The hydrothermal system at Yellowstone National Park has a relatively high amount of helium-3, and scientists consider this to be evidence that the Yellowstone hotspot originates deep within the mantle and has a relatively straight path up.
But it seems that the helium-4 has a more complicated journey to the surface. Researchers from the USGS have been collecting gas samples from across Yellowstone for a decade and performing chemical and isotopic analyses of the helium, carbon dioxide and other gases in those samples. The amount of helium-4 that’s being emitted in Yellowstone, they found, exceeds by several orders of magnitude the average amount they'd expect to find elsewhere.
Most of Yellowstone’s helium-4 is at least hundreds of millions—perhaps even billions—of years old, the scientists calculated. Given the amount of gas present, scientists conclude that it probably comes from rocks within very ancient crust known to be buried nearby. This super-old pocket of crust, from the Archaean eon, was formed more than 2.5 billion years ago and contains uranium and other radioactive materials that have been steadily decaying. That would have allowed high concentrations of helium-4 to build up underground. Then about two million years ago, the Yellowstone hotspot penetrated these ancient rocks, some of the oldest on the planet, and began releasing the helium-4 stored there along with helium-3 brought up from the mantle.
Helium 4 Atomic Number
This discovery probably qualifies as “kind of interesting” for any Yellowstone fans, but for scientists, there might be more important implications. Helium and other noble gases are used to estimate groundwater residence times—for example, scientists assume that the more helium-4 present in water, the longer that water has been sitting in the rocks surrounding it.
But the study of helium at Yellowstone shows that some of these assumptions—specifically helium-4 produced by the steady decay of elements found only within the rocks and sediments of the local aquifer—aren’t quite right. Helium can suddenly come into a system from unexpected places—a pocket of ancient rock, for instance, or an magma source—so the dates in past calculations, particularly those from aquifers in volcanic regions or near earthquake faults, might be way off because of that extra helium.
Scientists, though, are used to dealing with new data that changes long-held theories; that’s the nature of science, after all. Residents of the Yellowstone area, however, probably wish researchers would just hurry up and figure out whether or not the supervolcano that’s simmering below them and last erupted 640,000 years ago is going to blow again anytime soon.
Helium-4
Helium 4 Atomic Mass
Helium-4 is a non-radioactive isotope of the element helium. It is by far the most abundant of the two naturally occurring isotopes of helium, making up about 99.99986% of the helium on Earth. Its nucleus is identical to an alpha particle, and consists of two protons and two neutrons.Alpha decay of heavy elements in the Earth's crust is the source of most naturally occurring helium-4 on Earth. While it is also produced by nuclear fusion in stars, most helium-4 in the Sun and in the universe is thought to have been produced by the Big Bang, and is referred to as 'primordial helium'. However, primordial helium-4 is largely absent from the Earth, having escaped during the high temperature phase of Earth's formation. Radioactive decay from other elements is the source of most of the helium-4 found on Earth, produced after the planet cooled and solidified.Helium-4 makes up about one quarter of the ordinary matter in the universe by mass, with almost all of the rest being hydrogen.When liquid helium-4 is cooled to below 2.17 Kelvin it becomes a superfluid, with properties that are very unlike those of an ordinary liquid. For example, if superfluid helium-4 is kept in an open vessel, a thin film will climb up the sides of the vessel and overflow. In this state and situation, it is called a 'Rollin film'. This strange behavior is a result of the Clausius–Clapeyron relation, and cannot be explained by the current model of classical mechanics, nor by nuclear or electrical models - it can only be understood as a quantum mechanical phenomenon. The total spin of the helium-4 nucleus is an integer, and therefore it is a boson. The superfluid behavior is now understood to be a manifestation of Bose-Einstein condensation, which occurs only with collections of bosons.
